EUTROPHICATION OF LAKES
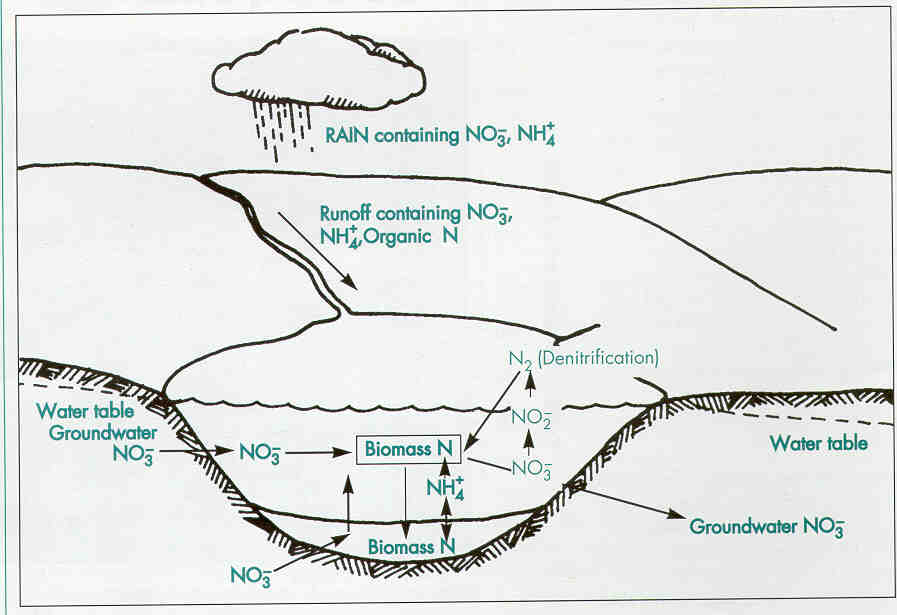
Nitrogen is second only to phosphorus as an important nutrient for plant and algae growth. A lake's nitrogen sources vary widely. Nitrogen compounds often exceed 0.5 mg/l in rainfall, so that precipitation may be the main nitrogen source for seepage and some drainage lakes.
RESULTS OF A 37-YEAR WHOLE-ECOSYSTEM EXPERIMENT
For years we have known that nitrogen and phosphorus enter lakes in chemical fertilizers, play a role in eutrophication- the process by which algal blooms, turbidity and oxygen depletion turn a lake into a dead zone, largely devoid of animal life. A recently published paper in the Proceedings of the National Academy of Sciences presents a new finding. In a 37 year experiment with a lake in northern Ontario, scientists demonstrated that controlling phosphorus in particular is the key to reversing eutrophication.
The experiment reveals the dynamics of eutrophication. Cyanobacteria, the blue-green algae responsible for the most common algal blooms, are nitrogen fixing- meaning that they get the nitrogen from the air. So elimination nitrogen from input into a body of water by controlling nitrogen does not solve the problem. "Nitrogen is important in determining which species grow," says Robert Hecky, a coauthor of the paper, and a professor of lake ecology at the University of Minnesota. "If we just control nitrogen, we'll merely shift the balance in favor of the species that can fix nitrogen, giving the same algal biomass. To bring dead zones back to life, phosphorus must be controlled".
TROPHIC STATUS OF LAKES
(Here are some excerpts from the New York State Citizens Statewide Lake Association Program, Scott Kishbaugh, New York State Department of Environmental Conservation from April 2007.)
Introduction: Lakes are dynamic and complex ecosystems. To better understand the water quality conditions of a lake scientists and concerned citizens in New York State conduct water quality monitoring programs. Water quality monitoring can assist scientists in determining whether a lake is undergoing changes that might impair the uses of the lake for drinking, swimming or recreation. As water quality changes, so too will the plants and animals that live there, and these changes in the food web also may affect water quality. Water quality monitoring provides a window into the numerous and complex interactions of lakes. Even the most extensive and expensive monitoring program cannot completely assess the water quality of a lake. However, by looking at some basic chemical, physical, and biological properties, it is possible to gain a greater understanding of the general condition of lakes.
Understanding Trophic States
All lakes and ponds undergo eutrophication, an aging process that involves stages of succession in biological productivity and water-quality. Limnologists (scientists who study freshwater systems) divide these stages into trophic states (Figure 1).
Figure 1 – Trophic State Classification
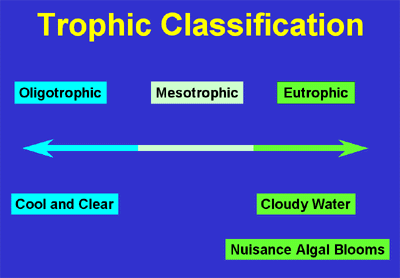
Source: Environmental Protection Agency
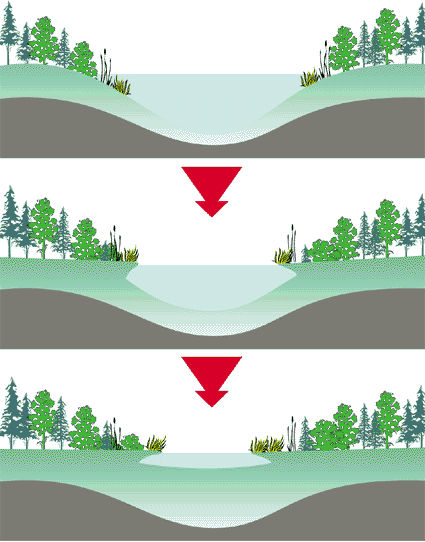
Each trophic state can represent a wide range of biological, physical, and chemical characteristics and any lake may be categorized within any of these trophic states. In general, the increase in productivity and decrease in clarity corresponds to an enrichment of nutrients, plant and animal life. Lakes with low biological productivity and clear water are considered oligotrophic. Highly productive lakes with low clarity are considered eutrophic . Lakes that are mesotrophic have intermediate or moderate productivity and clarity. It is important to remember that eutrophication is a natural process and is not necessarily indicative of man-made pollution. Over a period of hundreds of years a lake will eventually fill with sediment due to natural erosion processes. Streams carry sediments to lakes; trees and other vegetation along the water's edge drop their leaves and add nutrients to the lake. Over time this provides habitat for other vegetation until the lake changes from an open water system to a wetland. Figure 2 shows this natural eutrophication process.
Figure 2 – Natural Eutrophication
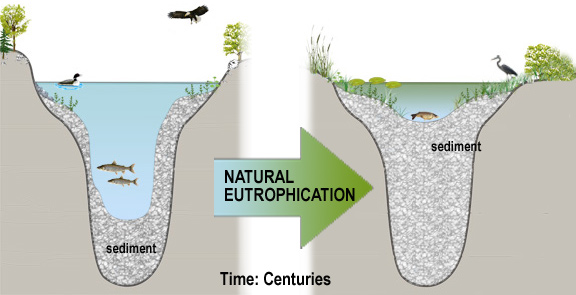
When human activities accelerate lake eutrophication, it is referred to as cultural eutrophication. Cultural eutrophication may result from shoreline erosion, agricultural and urban runoff, wastewater discharges or septic seepage, and other non-point source pollution sources. These can greatly accelerate the natural aging process of lakes, cause successional changes in the plant and animal life within the lake, shoreline and surrounding watershed, and impair the water-quality and value of a lake. They may ultimately extend aquatic plants and emergent vegetation throughout the lake, resulting in the transformation of the lake into a marsh, prairie, and forest. The extent of cultural eutrophication and the corresponding pollution problems can be signaled by significant changes in the trophic state over a short period (decades versus centuries). For most lakes in New York , cultural eutrophication represents the most significant source of pollutants and threat to water-quality. As a result, water-quality indicators related to eutrophication comprise the foundation of most water-quality monitoring programs.
Classifying a Lake 's Trophic Status
Trophic classifications are not interchangeable. People's perceptions of a lake may not coincide with the trophic status. Water quality degradation from the perspective of one user may contrast with the perception of favorable conditions by a different lake user. For example, a eutrophic lake may support an excellent warm-water fishery because it is nutrient rich, but a swimmer may describe that same lake as polluted because it is too weedy.
A lake's trophic state is important because it provides lake managers with a reference point to view changes in a lake's water quality and they begin to understand how these changes may cause use impairments (threaten the use of a lake for swimming, drinking water or fishing) .
Three important measures of eutrophication in most New York lakes are: total phosphorus, chlorophyll a (estimating the amount of algae), and Secchi disk transparency . Because these parameters are closely linked to the growth of weeds and algae, they provide insight into “how the lake looks” and its suitability for recreation and aesthetics.
Eutrophication
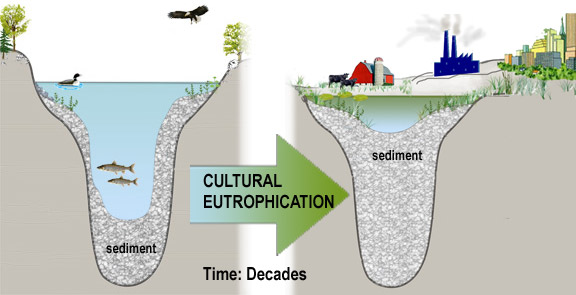
Eutrophication is a process that results from accumulation of nutrients in lakes or other water bodies. Eutrophication is a natural process, but can be greatly accelerated by human activities that increase the rate at which nutrients enter the water.
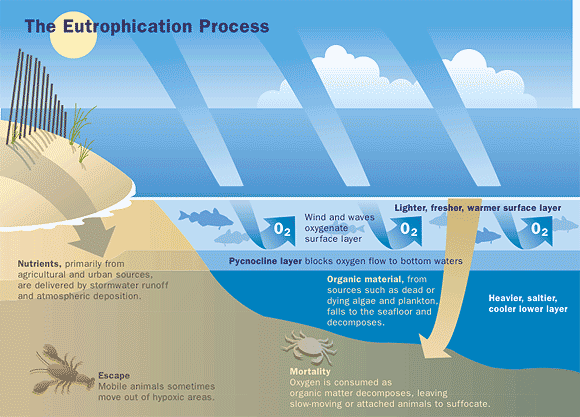
Algae growth is limited by the available supply of phosphorus or nitrogen, so if excessive amounts of these nutrients are added to the water, algae and aquatic plants can grow in large quantities. When these algae die, they are decomposed by bacteria, which use dissolved oxygen. This process is called "eutrophication." Dissolved oxygen concentrations can drop too low for fish to breathe, leading to fish kills. Excessive amounts of algae grow into scum on the water surface, decreasing recreational value and clogging water-intake pipes. Rapid decomposition of dense algae scums with associated organisms can give rise to foul odors.
In freshwater lakes and rivers, phosphorus is often the growth limiting nutrient, because it occurs in the least amount relative to the needs of plants. In estuaries and coastal waters, nitrogen is generally the growth limiting nutrient.
"Eutrophic" waters are characterized by high nutrient concentrations, resulting in high productivity of plant growth. Such waters are often shallow, with algal blooms and periods of oxygen deficiency. Slightly or moderately eutrophic water can support a complex web of plant and animal life. However, such waters are generally undesirable for drinking water and other needs. Waters with extreme nutrient concentrations are called "hypereutrophic."
"Oligotrophic" waters are characterized by extremely low nutrient concentrations, resulting in moderate plant productivity. Oligotrophic lakes are those low in nutrient materials and consequently poor areas for the development of extensive aquatic plants and animals. Such lakes are often deep, with sandy bottoms and very limited plant growth, but with high dissolved-oxygen levels.
Some scientists have categorized trophic status according to phosphorus concentration. Lakes with phosphorus concentrations below 0.010 mg/L are classified as oligotrophic, phosphorus concentrations between 0.010 and 0.020 mg/L are indicative of mesotrophic lakes, and eutrophic lakes have phosphorus concentrations exceeding 0.020 mg/L (Muller and Helsel, 1999)
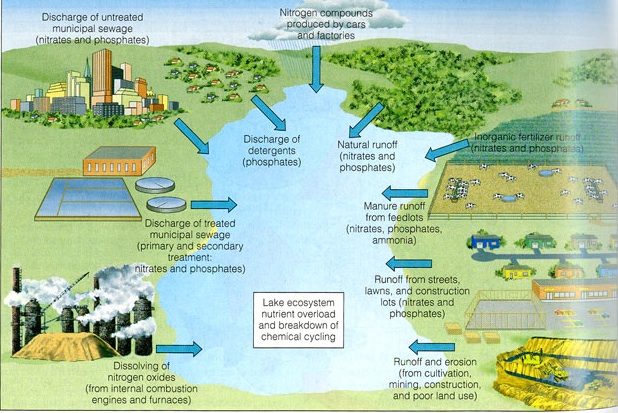
Cultural Eutrophication
GENERAL INFORMATION ON PHOSPHORUS
by Sheila Murphy
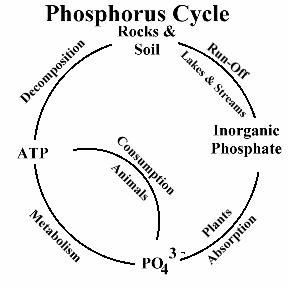
Phosphorus is a nutrient required by all organisms for the basic processes of life. Phosphorus is a natural element found in rocks, soils and organic material. Phosphorus clings tightly to soil particles and is used by plants, so its concentrations in clean waters is generally very low. However, phosphorus is used extensively in fertilizer and other chemicals, so it can be found in higher concentrations in areas of human activity. Many seemingly harmless activities added together can cause phosphorus overloads. Phosphorus exists in water in either a particulate phase or a dissolved phase. Particulate matter includes living and dead plankton, precipitates of phosphorus, phosphorus adsorbed to particulates, and amorphous phosphorus. The dissolved phase includes inorganic phosphorus and organic phosphorus. Phosphorus in natural waters is usually found in the form of phosphates (PO4-3). Phosphates can be in inorganic form (including orthophosphates and polyphosphates), or organic form (organically-bound phosphates).
Organic phosphate is phosphate that is bound to plant or animal tissue. Organic phosphates are formed primarily by biological processes. They are contributed to sewage by body waste and food residues, and also may be formed from orthophosphates in biological treatment processes or by receiving water biota. Organic phosphates may occur as a result of the breakdown of organic pesticides which contain phosphates. They may exist in solution, as loose fragments, or in the bodies of aquatic organisms. Inorganic phosphate is phosphate that is not associated with organic material. Types of inorganic phosphate include orthophosphate and polyphosphates. Orthophosphate is sometimes referred to as "reactive phosphorus." Orthophosphate is the most stable kind of phosphate, and is the form used by plants. Orthophosphate is produced by natural processes and is found in sewage. Polyphosphates (also known as metaphosphates or condensed phosphates) are strong complexing agents for some metal ions. Polyphosphates are used for treating boiler waters and in detergents. In water, polyphosphates are unstable and will eventually convert to orthophosphate.
Phosphates are not toxic to people or animals unless they are present in very high levels. Digestive problems could occur from extremely high levels of phosphate. In freshwater lakes and rivers, phosphorus is often found to be the growth-limiting nutrient, because it occurs in the least amount relative to the needs of plants. If excessive amounts of phosphorus and nitrogen are added to the water, algae and aquatic plants can be produced in large quantities. When these algae die, bacteria decompose them, and use up oxygen. This process is called eutrophication. Dissolved oxygen concentrations can drop too low for fish to breathe, leading to fish kills. The loss of oxygen in the bottom waters can free phosphorus previously trapped in the sediments, further increasing the available phosphorus.
Measurement of Phosphorus
There are several forms of phosphorus which can be measured. Total phosphorus (TP) is a measure of all the forms of phosphorus, dissolved or particulate, that are found in a sample. Soluble reactive phosphorus (SRP) is a measure of orthophosphate, the filterable (soluble, inorganic) fraction of phosphorus, the form directly taken up by plant cells.
Both phosphorus and orthophosphate are often measured using a colorimetric method, which means the color of treated sample reflects the concentration of the parameter. If total phosphorus is being measured, all forms of phosphorus are converted to dissolved orthophosphate with acid, persulfate, and heat. A chemical is then added to the water sample. The darker the color of the sample becomes, the more phosphorus present. This test can be done visually, comparing the treated sample to a set of reference colors. However, it is more accurate to use an electronic colorimeter, which uses a light source and a photodetector to find the concentration based on how much light is absorbed by the sample.
Factors Affecting Phosphorus Concentrations
Wastewater and Septic System Effluent
Domestic and industrial sewage are very important sources of phosphorus to surface water. Organic phosphates are formed primarily by biological processes. They are contributed to sewage by body waste and food residues. Phosphorus is essential in metabolism so is always present in animal waste. Orthophosphates and polyphosphates can be contributed by detergents, as discussed below.
Detergents
Orthophosphates and certain polyphosphates are major constituents of many commercial cleaning preparations. In the 1950s and 1960s, sodium phosphate was used often as a "builder" in households detergent to increase cleaning power. The extensive use of detergents led to major eutrophication problems, and in the 1960s efforts were made by governments, detergent manufacturers, and consumers to reduce the use of phosphates in detergents. As a result, phosphorus concentrations in many streams and lakes decreased. This was due to limits on the phosphate content of detergent, and also additional treatment used in waste water treatment plants to remove phosphorus. Many states have a ban on phosphates in detergents.
Fertilizers
Fertilizers generally contain phosphorus in the form of orthophosphate. Phosphate is not very mobile in soil; it tends to remain attached to solid particles rather than dissolving in water. However, if too much fertilizer is applied, the phosphates are carried into surface waters with storm runoff and also with melting snow. Soil erosion of fertilized fields and lawns can also carry a considerable amount of particulate phosphate to streams.
Animal Waste
Phosphorus is essential in metabolism, so is present in animal waste. Therefore, phosphate runoff can be an issue in waters near cattle feedlots, hog farms, dairies, and barnyards.
Development/Paved Surfaces
Development can cause soil erosion, which will release phosphorus. If swamps and wetlands are drained for development, phosphorus that was buried can be exposed. During the building phase, and after everything has stabilized, phosphorus concentrations in stormwater can increase because natural filters such as trees, shrubs, and puddles have been eliminated.
Industrial Discharge
Polyphosphates are often added to water to prevent iron oxides or calcium carbonates from forming. If this water is released to streams or lakes, polyphosphates can enter the water body, and will convert to orthophosphate.
Phosphate Mining
Phosphate mining, concentrating, and processing are sources of phosphate to rivers in some areas. The most common phosphorus-containing mineral is apatite (Ca5F(PO4)3). There are no significant sources of phosphate minerals in the Boulder Creek Watershed, so this is not a problem in our area.
Drinking Water Treatment
Small amounts of orthophosphates or certain polyphosphates are added to some water supplies during treatment.
Forest Fires
Forest fires can cause soil erosion, which will release phosphorus bound to soil particles.
Synthetic Materials
Organophosphates are commonly used as construction materials, flame retardant and plasticizers. Reduced forms of phosphorus are present in certain synthetic organic chemicals, including some that are used in insecticides.
Water Quality Standards and Other Criteria Regarding Phosphorus
No national or state criteria have been established for concentrations of phosphorus compounds in water. However, to control eutrophication, the EPA makes the following recommendations: total phosphate should not exceed 0.05 mg/L (as phosphorus) in a stream at a point where it enters a lake or reservoir, and should not exceed 0.1 mg/L in streams that do not discharge directly into lakes or reservoirs (Muller and Helsel, 1999). Phosphate levels greater than 1.0 mg/L may interfere with coagulation in water treatment plants. As a result, organic particles that harbor microorganisms may not be completely removed before distribution.
EUTROPHICATION (Nutrient Pollution)
Natural eutrophication is the process by which lakes gradually age and become more productive. It normally takes thousands of years to progress. However, humans, through their various cultural activities, have greatly accelerated this process in thousands of lakes around the globe. Cultural or anthropogenic "eutrophication" is water pollution caused by excessive plant nutrients. During the 1960's, Lake Erie was undergoing rapid cultural eutrophication and was the subject of much concern. The ELA was established in 1968 to experimentally investigate this problem. Between June, 1969 and May, 1976, it was virtually the sole focus of whole-ecosystem experimental studies at the ELA.
Humans add excessive amounts of plant nutrients (primarily phosphorus, nitrogen, and carbon) to streams and lakes in various ways. Runoff from agricultural fields, field lots, urban lawns, and golf courses is one source of these nutrients. Untreated, or partially-treated, domestic sewage is another major source. Sewage was a particular source of phosphorus to lakes when detergents contained large amounts of phosphates. The phosphates acted as water softeners to improve the cleaning action, but they also proved to be powerful stimulants to algal growth when they were washed or flushed into lakes.
The excessive growth, or"blooms", of algae promoted by these phosphates changed water quality in Lake Erie and many other lakes. These algal blooms led to oxygen depletion and resultant fish kills. Many native fish species disappeared, to be replaced by species more resistant to the new conditions. Beaches and shorelines were fouled by masses of rotting, stinking algae. A means to control this problem became a paramount need.
Using small, natural lakes as experimental systems, scientists at the ELA were able to add various combinations of nutrients and determine which of the major plant nutrients (carbon, nitrogen, phosphorus) was the key to controlling cultural eutrophication in lakes. Over a number of years, seven different ELA lakes (227, 304, 302, 261, 226, 303, 230) were experimentally fertilized in various ways. Two of these lakes (227 and 226) were particularly important in demonstrating that phosphorus was the key nutrient for the control of eutrophication.
Studies of gas exchange and internal mixing in ELA lake 227 during the early 1970's clearly demonstrated that algae in lakes were able to obtain sufficient carbon dioxide, via diffusion from the atmosphere to the lake water, to support eutrophic blooms. Other studies in the same lake demonstrated that certain blue green "algae" (Cyanophytes or Cyanobacteria) were able to "fix" nitrogen that had diffused naturally into the lake from the air, thereby making the nitrogen available for supporting algal growth.
ELA Lake 226 was the site of a visually spectacular experiment. The lake was divided into two approximately equal portions using a plastic divider curtain. Carbon and nitrogen were added to one half of the lake, while carbon, nitrogen and phosphorus were added to the other half. For eight consecutive years, the side receiving phosphorus developed eutrophic algal blooms, while the side receiving only carbon and nitrogen did not (see photo, below). However, after only two years, this experiment convinced even the skeptics that phosphorus is the key nutrient. A multi-billion dollar phosphate control program was soon instituted within the St. Lawrence Great Lakes Basin. Legislation to control phosphates in sewage, and to remove phosphates from laundry detergents, was part of this program.

View from above Lake 226 divider curtain in August 1973. The bright green colour results from bluegreen algae (Cyanobacteria), which are growing on phosphorus added to the near side of the curtain. By the mid-1970's, North American interest in eutrophication had waned. However, this "nutrient pollution" problem remains the number one water quality problem worldwide. Eutrophication research at the ELA has continued in Lake 227, albeit on a much reduced scale. After more than three decades, ELA scientists continue to unravel the details of algal responses to nutrients and the food chain effects that accompany them.

Aerial view of Lake 227 in 1994. Note the bright green colour caused by algae stimulated by the experimental addition of phosphorus for the 26th consecutive year. Lake 305 in the background is unfertilized. (photo by Karen Scott.)
Preamble
Man-made accelerated eutrophication of inland waters in OECD Member countries can generally be viewed as an undesirable degradation of the environment resulting in a deterioration of water quality which interferes with most of the beneficial uses of waters; it is causing, in many cases, significant economic losses.
The impact of eutrophication on recreation and tourism is probably the most sensitive area for the public. It may severely alter the recreational value of many water bodies and impair related activities (swimming, fishing, etc.) as a result of the objectionable aspect of the waters, such as reduced transparency, odour, and increased incidence of stinging insects, swimmer's itch, etc. Both social impacts and economic losses may be important and make eutrophication control necessary.
The impact of eutrophication on drinking water supply may be serious. For a number of reasons it generally reduces its final quality and may diminish its safety. Furthermore, it makes its preparation more difficult and costly.
The problems encountered include: rapid clogging of filters by diatoms and other algae; disturbance of floculation treatment by organic substances; persistent and unpleasant taste and odour (e.g. geosmine); abnormal concentration of substances such as manganese, iron and ammonia giving rise to colour or other disturbances; risk of increased bacterial growth in drinking water due to the fouling of the distribution networks and the nutrient content.
Furthermore, because of the high content of organic substances in eutrophied waters and some of the problems listed above (taste, ammonia, regrowth of organisms, etc.) these waters are often extensively chlorinated during treatment as well in the initial transportation and final distribution networks. High levels of both chlorine and organic substances lead to significant concentrations of organochlorinated compounds in drinking water, and these substances are now considered to be potentially hazardous for human health (carcinogenic risk). Waters for potable use should thus be protected from eutrophication.
EUTROPHICATION OF WATERS (OECD)
Monitoring, Assessment and Control
Research of the Organization for Economic Co-Operation and Development (OECD)
Introduction
The OECD lakes ranged from "pond-size" lakes to the Great North American Lakes. The momentum initiated by the International Biological Programme in 1964 was maintained. The information available was broad enough to establish the general statistical behaviour of lakes with respect to nutrient load and trophic response. It should be noted, however, that subtropical (in USA) and Arctic lakes (including high Alpine) were poorly represented, and saline, closed basin lakes were not represented at all in the programme. The OECD study was restricted mainly to lakes of the temperate zone. The final report is a synthesis of the main results of the OECD Cooperative Programme on Eutrophication under the Chairman of the Technical Bureau, Dr. Richard Vollenweider. It is the outcome of several years' concerted effort by 18 Member countries. The objectives were to establish, through international cooperation, a basis for eutrophication control of inland waters (lakes and reservoirs in particular), and to develop better guidelines for fixing nutrient load criteria compatible with water use objectives.
This report is both complementary and supplementary to the four Regional Project Reports published as follows. A fifth report (the Canadian contribution) has been developed which tests the OECD results on bodies of waters not included in the analytical part.
Summary and Conclusions:
The objectives of the programme have been largely achieved, and applicaion of the results to practical control of eutrophication is possible. However, it is recommended that the results be handled with caution and not applied to cases which lie outside the ranges and situations covered by the programme. The main control strategy is reduction of the external load. In cases where such a reduction to the required tolerance level is impracticable, or impossible (as e.g. in situations of intensive use of the catchment system), management measures other than nutrient reduction have to be employed. Such alternative measures, however, can only be defined using advanced modelling techniques. Notwithstanding the positive achievements of the programme, eutrophication remains a very complex problem and numerous questions are still unanswered. Although nitrogen or some other factor may be in some particular instances outweigh the role of phosphorus as the limiting factor, most attention here is based on phosphorus control; however, where the term "nutrients" appears, both phosphorous and nitrogen may be considered in order to include potential cases of nitrogen limitation. It is safer and generally more economic to take early preventive measures to control eutrophication than to develop curative strategies later when water quality has already deteriorated.
Water Resource Planning
The results of the OECD programme have shown that: in most cases, phosphorus is the factor which determines the development of eutrophication; even when another nutrient such as nitrogen is (occasionally or normally) the limiting factor, phosphorus may still be made to play the role of limiting factor through appropriate control. Control of point sources of pollution from municipalities and industries is usually given priority as it is generally the most cost-effective measure. After reduction of phosphorus from point sources, the relative role of phosphorus from diffuse sources will increase. This means that measures against diffuse sources may become necessary if improvement of water quality cannot be achieved by further elimination of phosphorus point sources. Diffuse source control is more difficult to achieve. Yet, in many cases, effective prevention of eutrophication, or restoration of eutrophied waters cannot be achieved without such control.
Therefore, the improvement of all aspects of agricultural practices which contribute nutrients to water bodies should be encouraged, with special reference to:
- control of waste from intensive animal husbandry;
- control of the dose, period and methods of fertiliser application in order to achieve minimal loss and optimum up-take by crops;
- control of erosion and run-off from tillage land and from forestry operations;
- control of over-irrigation (fertiliser leaching to ground waters).
Attention should also be given to urban run-off and storm overflows, which normally by-pass conventional treatment plants. A more integrated approach may be necessary, and should include modifications to system design standards, source controls, sewer separation, flow detention, and overflow treatment. In certain situations nutrient contributions from septic tanks may be important. Potential contributions from such sources should be carefully assessed, although no generally applicable methodology exists to control septic tank leaching. However, when septic tank effluent is dispersed by tiled drains in a field, a high degree of phosphorus removal can be achieved for a considerable time, especially with certain types of soils.
Eutrophication cannot really be controlled by herbicides and algicides. As the use of these products presents a hazard, they should only be considered as an interim measure to alleviate symptoms in exceptional cases and strictly limited environments. Although the OECD Programme was designed to control the mainly negative effects of eutrophication, there may be exceptional cases where an increase in the trophic level can have limited beneficial aspects (e.g. for increasing the population of certain fish species). The potential benefits are, however, rapidly outweighed by the negative effects on all uses as trophic levels increase. Greater attention should be given to the economic use and the promotion of by-products from water pollution and eutrophication control measures. Instances include: low cost energy gains from sludge digestion (bio-gas); potential use of sludge as fertiliser products; irrigation with waste water ("fertirrigation"); harvesting of primary products from controlled lagooning, etc
Nutrient Load Assessment
For practical purposes, the following break-down scheme is recommended for estimating the total load.
External:
- the phosphorus and nitrogen load via the tributaries - the point and diffuse sources load which enter the lake through the shores;
- the phosphorus and nitrogen load which falls on to the surface of a lake as wet or dry precipitation;
Internal:
- phosphorus and nitrogen which re-enter from sediments. The net result of the interchange of nutrients between water and bottom sediments can be estimated by making nutrient balances covering relevant periods of time.